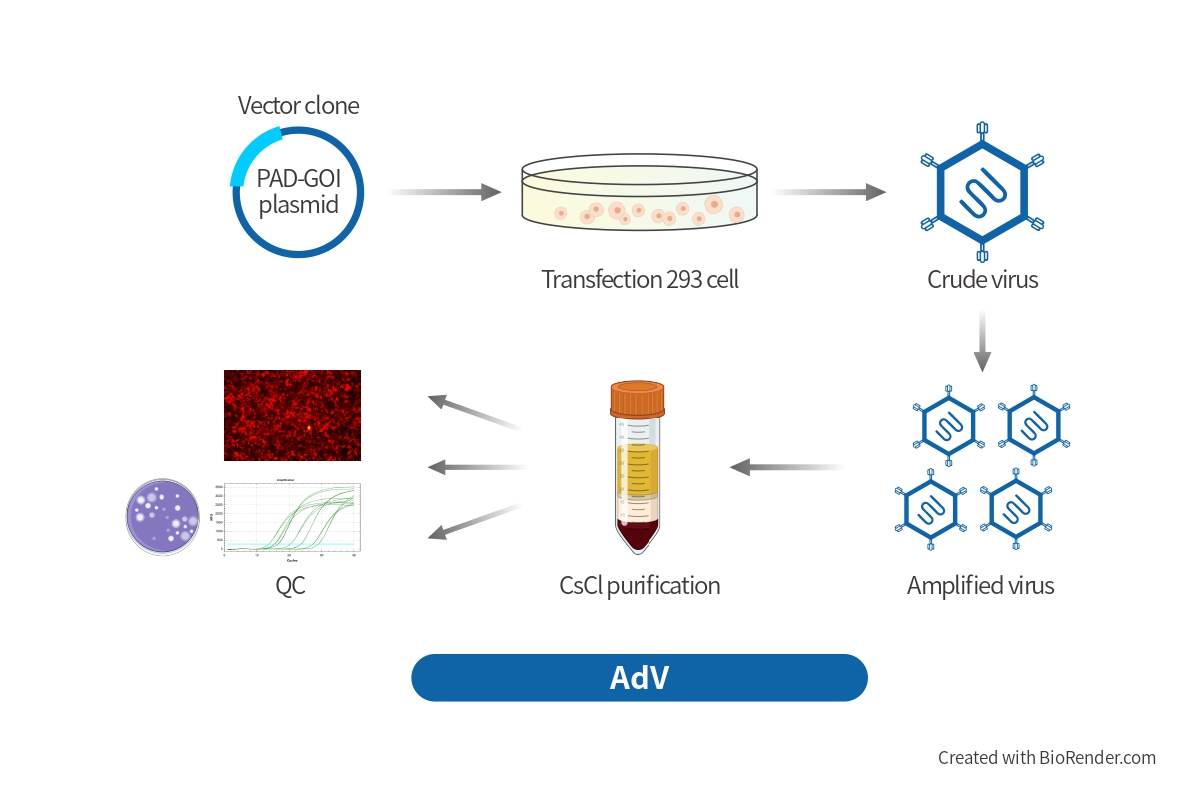
Custom Adenovirus Vector Design
We also provide Cloning Services to allow you to fully customize your order at a reasonable cost and with a quick turn around time, include over-express any genes (wildtype, mutant or synthetic) or to silence/knock-down/knock-out/knock-in any genes. Please contact us with your project details and our team will get in touch as soon as possible with an estimated price and projected turnaround time. You can also visit our Molecular Cloning page for more information. If you already have the ORF in plasmids, you just need to send the min- plasmid to us, then we will clone in Adenovirus vector and package it.
Recombinant Lentivirus packaging services
BrainVTA provides the complete AdV vectors and AdV Expression Systems that can be used to express human/mouse/rat ORFs, lncRNAs, circRNAs, shRNAs, CRISPR/gRNA in vitro and in vivo studies. Our optimized adenoviral vector production and strict quality control systems provide customers a high titer of functional recombinant adenoviral vectors. Viral titers are determined by TCID50 method, which is the most accurate way to measure the titer of adenovirus.
.jpg)
Adenovirus packaging Services FAQs
-
1. What is the required biosafety level for using recombinant adenoviruses?
The recombinant adenoviruses we produce are replication deficient due to deletions in the E1 and E3 regions. According to references issued by the NIH Office of Biosafety, recombinant human adenoviruses are classified as biosafety level II for agents considered ordinary potential harm. You need a BL-2 level facility to work with them. It should be noted that cell culture facilities in most institutes are certified as BL-2 level.
-
2. What’s the optimal concentration of viruses that I should use for infection?
The appropriate amount of active/infectious viruses used for infecting cells is very important for the outcome of your experiments. If not enough virus is used, you will not reach 100% infection. If too much is used, it will result in cytotoxicity or other undesired effects. You should use the minimum viral concentration that will result in 100% gene delivery. This optimal concentration differs dramatically between cell types. To determine this optimal viral concentration, you could conduct pilot testing in your system by using marker adenoviruses, such as Ad-CMV-ß-gal.
-
3. What are the differences between Viral Particle (VP), Plaque Formation Unit (PFU) and Infectious Unit (IFU)? Which one of these better reflects the amount of active virus used?
Viral Particles (VP) represent the total number of both live and dead viral particles. Due to variations in virus preparations, the ratio of live/dead varies significantly. Therefore, VP does not reflect the amount of active virus in the preparation.
Plaque Formation Unit (PFU) represents the number of infectious or live viruses. It reflects the amount of working viruses in the preparation. Infectious Unit (IFU) is equivalent to PFU.
For most virus preparations , the VP/PFU ratio is 20:1 to 50:1. Using Viral Particles (VP) as measurement will result in significant variations in the amount of actual live viruses present, whereas using IFU or PFU as the viral unit will give more consistent outcomes. -
4. Can I make a stable cell line with adenovirus?
No, adenoviral vectors can only be used transiently
-
5. Are adenoviruses replication deficient?
Typically yes, because the early genes necessary for replication have been deleted from the shuttle vector. Early gene E1 is provided by the transfected cell line (either 293 or 911). To avoid creating replication competent virus, you should not serially propagate your virus as the chance of recombination events creating replication-competent virus increases with each round of amplification.
-
6. What are the recommended storage conditions of recombinant adenoviruses?
For long-term storage, the virus should be kept at -80°C, especially after CsCl or chromatography purification. At -80°C, the virus should be stable for 6 months to a year (and in some cases, up to 2 years).
-
7. What is the capacity of cloning into the adenovirus as an expression system?
The cloning capacity for the transgene, by using (DE1/E3) adenovirus type 5, is about 8 Kb in length.
References
- Dang T, Duan W Y, Yu B, et al. Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development[J]. Molecular Psychiatry, 2017.
- . Cetin, A. and E.M. Callaway, Optical control of retrogradely infected neurons using drug-regulated "TLoop" lentiviral vectors. J Neurophysiol, 2014. 111(10): p. 2150-9.
- Wei, Y., et al., Lentiviral vectors enveloped with rabies virus glycoprotein can be used as a novel retrograde tracer to assess nerve recovery in rat sciatic nerve injury models. Cell Tissue Res, 2014. 355(2): p. 255-66.
- Parr-Brownlie, L.C., et al., Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front Mol Neurosci, 2015. 8: p. 14.
- Parr-Brownlie, Louise C., et al. "Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms." Frontiers in Molecular Neuroscience. 8(2015).
- De Juan-Sanz J , Holt G T , Schreiter E R , et al. Axonal Endoplasmic Reticulum Ca 2+, Content Controls Release Probability in CNS Nerve Terminals[J]. Neuron, 2017, 93(4):867-881.e6.
- Sakurai K , Zhao S , Takatoh J , et al. Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit[J]. Neuron, 2016:S0896627316307164.
- Yao X H , Wang M , He X N , et al. Electrical coupling regulates layer 1 interneuron microcircuit formation in the neocortex[J]. Nature Communications, 2016, 7:12229.