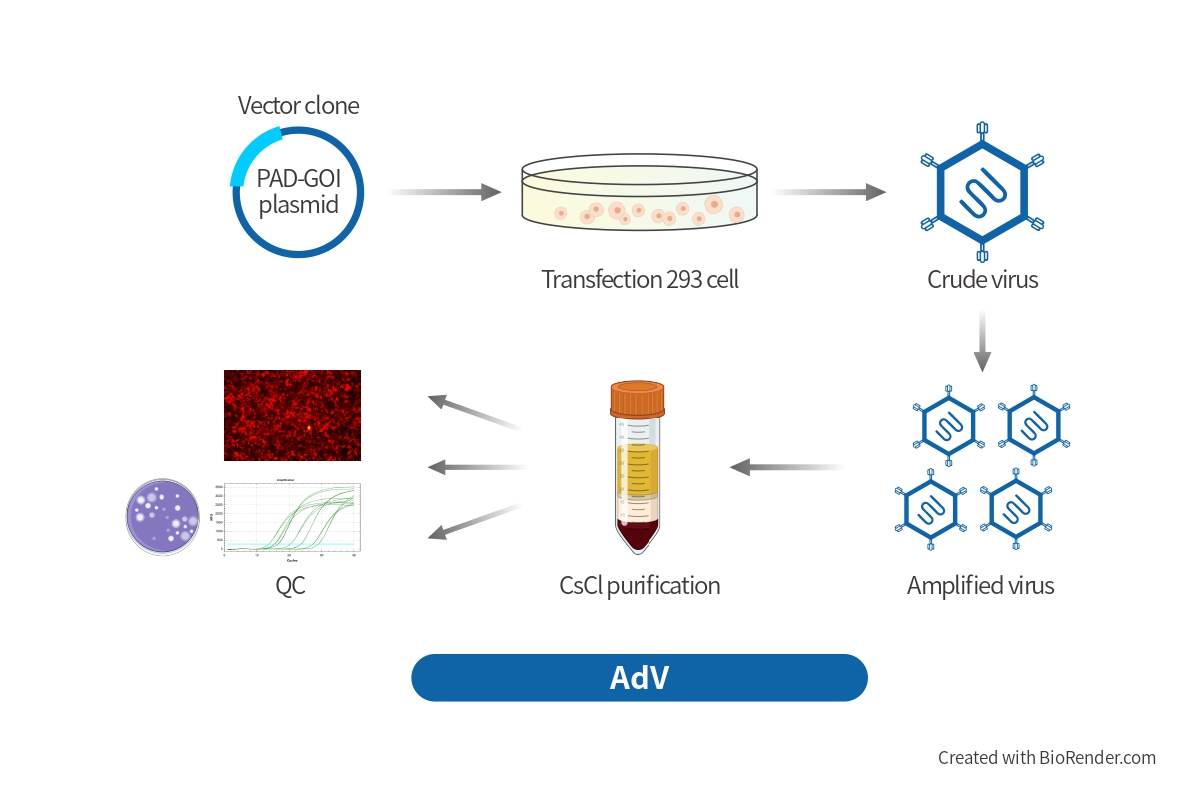
BrainVTA's AAV-HBV platform has a team of experienced scientists with deep knowledge of AAV-HBV production. We provide AAV particles with HBV genome DNA to establish the mouse models of HBV infection and replication. The model has three advantages: long termtransduction, fast HBV modeling, and stable expression of hepatitis B antigen, which accelerates the process of hepatitis B research and new drug development.
Applications
● Establish of hepatitis B virus infection model
● Basic research on hepatitis B virus infection
● Development of antiviral drugs
Available AAV-1.3XHBV Particles
For other genotypes or variants, customized service is available!
| Cat# | Name | Description | Price |
|---|---|---|---|
| PT-0717 | rAAV8-HBV1.3-mer WT replicon | Genotype D, Serotype ayw | Inquiry |
| PT-7351 | rAAV8-1.3HBV(B-adw) | Genotype B, Serotype adw | Inquiry |
| PT-7350 | rAAV8-1.3XHBV(C-adr) | Genotype C, Serotype adr | Inquiry |
| PT-0717 | rAAV8-HBV1.3-gtF | Genotype F | Inquiry |
| PT-0717 | PT-0717-HBsAg2mut | Genotype D, Serotype ayw, HBe Ag Negative | Inquiry |
Effect Verification
rAAV8-1.3xHBV was injected in C57 mice by via tail vein with the total virus is 2.3x1010vg, 5x1010vg and 10x1010vg, and the volume is 200uL, respectively.The contents of HBsAg, HBeAg and DNA markers of hepatitis B virus in serum were detected by orbital blood collection and observed continuously for 7 weeks.
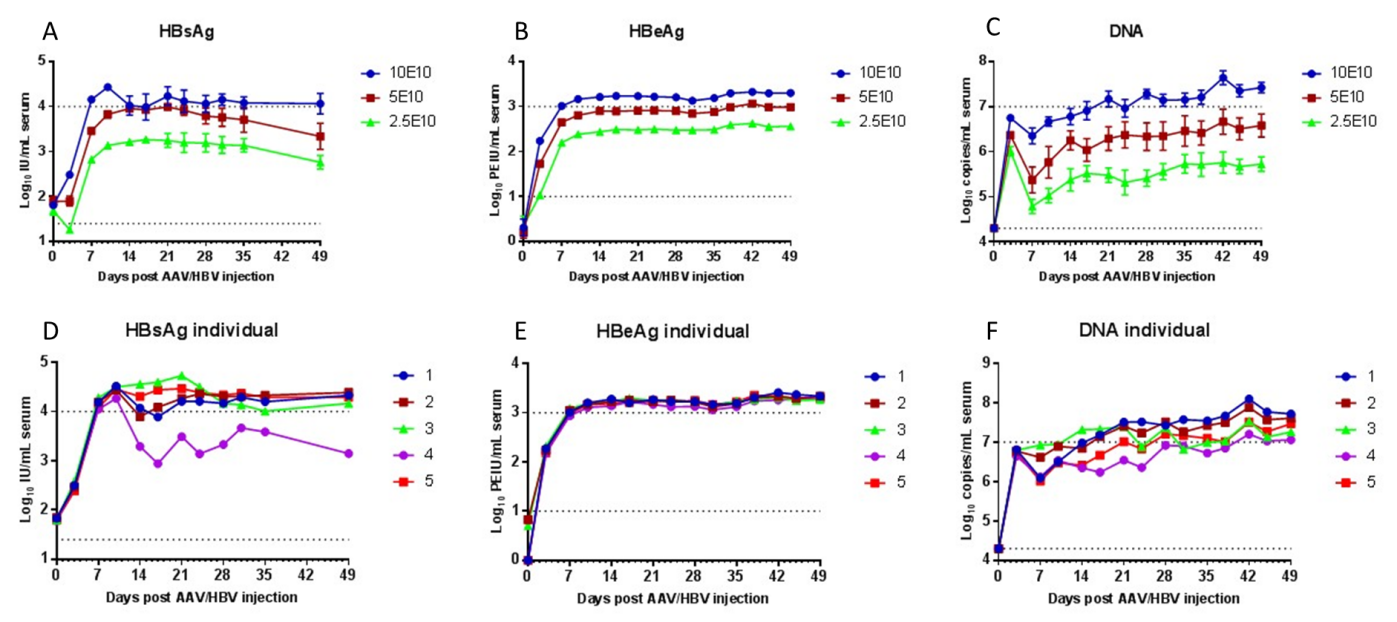
Fig.2: Effect test of mouse model of hepatitis B viruss established by AAV-1.3xHBV. ( A-C, Different doses of virus were injected by the tail vein, and 5 balance tests for each dose. The content of hepatitis B virus marker in serum was detected. D-F, Detection results of hepatitis B virus markers in the highest dose group with the five mice.)
References
- Global Hepatitis Report, 2017. WHO
- Chronic Hepatitis B Virus Infection: Developing Drugs for Treatment Guidance for Industry. 2018 FDA
- Christoph Seeger, and William S. Mason. Hepatitis B Virus Biology. Microbiology and Molecular Biology Reviews (2000). 51-68.
- Haifeng Wang, Seahee Kim, and Wang-Shick Ryu. DDX3 DEAD-Box RNA Helicase Inhibits Hepatitis B Virus Reverse Transcription by Incorporation into Nucleocapsids. Journal of General Virology (2009). 5815-5824.
- Man-Young Cha, Dong-Kyun Ryu. Stimulation of hepatitis B virus genome replication by HBx is linked to both nuclear and cytoplasmic HBx expression. Journal of General Virology (2009). 90:978-986.